Ampicillinsulbactamupdate
-
Rating
-
Date
March 2018 -
Size
821.2KB -
Views
518 -
Categories
Transcript
Drug Evaluation
Ampicillin-sulbactam: an update on the use of parenteral and oral forms in bacterial infections
1. 2. 3. 4. Expert Opin. Drug Metab. Toxicol. Downloaded from informahealthcare.com by HINARI on 10/08/10 For personal use only. 5. 6. 7. 8. Introduction Chemistry Pharmacokinetics, in vitro activity, resistance Clinical trials Infections due to Acinetobacter spp Safety and tolerability Conclusions Expert opinion
Alex P Betrosian† & Emmanuel E Douzinas
Athens University, Evgenidion Hospital, 3rd Department of Critical Care, 20 Papadiamantopoulou Street, 11528, Athens, Greece
Ampicillin-sulbactam has a wide range of antibacterial activity that includes Gram-positive and Gram-negative aerobic and anaerobic bacteria. However, the drug is not active against Pseudomonas aeruginosa and pathogens producing extended-spectrum β-lactamases. The combination could be considered particularly active against Acinetobacter baumannii infections due to the intrinsic activity of sulbactam. The drug is indicated as empirical therapy for a broad range of community acquired infections supervened in adults or children and is effective in either parenteral (ampicillin-sulbactam) or oral (as a mutual prodrug sultamicillin) form. In clinical trials, sultamicillin has proved clinically and bacteriologically effective in adults and children against a variety of frequently encountered infections, including mild upper and lower respiratory tract infections, urinary tract infections, diabetic foot and skin and soft tissue infections. Furthermore, adverse effects rarely occur with the diarrhoea to represent the most commonly reported. The parenteral ampicillin-sulbactam is indicated for community infections of mildto-moderate severity acquired infections such as intra-abdominal or gynecological. Moreover, it seems to represent the alternative of choice for the treatment of A. baumannii infections for carbapenem-resistant strains in the nosocomial setting. Thus, ampicillin-sulbactam remains a valuable agent in the physician’s armamentarium in the management of adult and pediatric infections.
Keywords: Acinetobacter baumannii, bacterial infections, ampicillin-sulbactam, sultamicillin Expert Opin. Drug Metab. Toxicol. (2009) 5(9):1099-1112
1. Introduction
In the era of global emergence and spread of bacterial resistance and in the absence of development of new effective antimicrobial agents, the correct use of the currently available antibiotics, in particular the penicillins, is of great significance. After the introduction and the widespread use of β-lactam antibiotics, many organisms initially susceptible to penicillins and derivatives developed resistance due to the ability to produce β-lactamases enzymes that hydrolyze the β-lactam ring, and, therefore, to destroy the antimicrobial activity of the antibiotics [1]. A highly effective and proven approach for tackling β-lactamase-mediated resistance to β-lactams is the use of β-lactam/β-lactamase-inhibitor combinations [2]. Such combinations have gained great success and have proven to be among the most effective antibiotic strategies [3]. Of the many β-lactamase inhibitors that have been evaluated, three inhibitors (sulbactam, tazobactam and clavulanic acid) are currently in clinical use in various combinations, including ampicillin-sulbactam, cefoperazone/ sulbactam, piperacillin/tazobactam, amoxicillin/clavulanic acid and ticarcillin/ clavulanic acid [4]. Sulbactam is also available as a single agent in some countries.
10.1517/17425250903145251 © 2009 Informa UK Ltd ISSN 1742-5255 All rights reserved: reproduction in whole or in part not permitted
1099
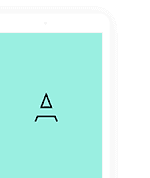
34 l/kg for sulbactam.1 Absorption and distribution Ampicillin and sulbactam is an injectable combination consisting of the semisynthetic antibiotic ampicillin and the β-lactamase inhibitor sulbactam sodium for intravenous (i. This problem has been overcome with the combination of ampicillin and sulbactam in one oral prodrug. 6-aminopenicillanic acid with the following chemical formula C16H18N3NaO4S. The therapeutic efficacy of parenteral and oral formulations of ampicillin-sulbactam has been demonstrated in a wide variety of infections.11. respectively) every 6 h with sulbactam usually not exceeding 4 g/day./ intramuscular dosing schedule and an optimal application form of 3 – 4 h infusion [8]. Sultamicillin is absorbed readily (> 80% bioavailability) leading to high serum concentrations of both agents. Peak plasma concentrations of both sulbactam and ampicillin are proportional to dose. have been demonstrated in cerebrospinal fluid with sulbactam to increase ampicillin’s penetration. [15] found increased bioavailability of ampicillin co-administered with sulbactam (orally and parenterally) when compared with amoxicillin plus clavulanic acid and concluded that the effect of sulbactam in increasing the oral bioavailability of ampicillin is greater than the effect of clavulanic acid on the oral bioavailability of amoxicillin. which exceed the MICs of important bacterial pathogens. Although the ratio of ampicillin:sulbactam differs between parenteral and oral forms (2:1 versus 1:1). sultamicillin. 3. attaining the level of 28%. This article aims primarily to concentrate on studies dealing with the treatment of various infections by the two available forms (parenteral and oral) of the ampicillin/sulbactam combination. It is a mutual prodrug that was developed to overcome the poor oral absorption of sulbactam. Pharmacokinetics. The average adult daily dose for the parenteral form depends on infection severity and ranges from 1. Ampicillin sodium is derived from the penicillin nucleus. Since then. Toxicol. Drug Metab. Sultamicillin (formerly known as CP-49. In a study with human volunteers. In two studies in which 500 mg of sultamicillin was compared with 500 mg of ampicillin alone.32 l/kg for ampicillin and 0. with the sulbactam oral absorption being very poor [10]. Chemically. particularly in the presence of inflamed meninges [16]. that is.5 g (1 g ampicillin plus 0. Both agents have similar time-to-peak plasma concentrations. gynecological infections. It is a double ester linkage of sulbactam with ampicillin. The protein binding of ampicillin and sulbactam in serum is similar. (2009) 5(9) . Information for the treatment of infections of milder severity comes from the use of the oral form of the drug. The pediatric dosage of the combination is 200 mg ampicillin/100 mg sulbactam per kilogram of body weight/day. Chemistry pharmacokinetics of sulbactam and vice versa [11]. However.5 g sulbactam) to 3 g (2 g/1 g.14].3-dimethyl-4. but tissue drug concentrations were not measured [12]. It is chemically designated as [(2R)-3. high concentrations have been reported in costal cartilage [17] and middle-ear fluid. High body tissue and fluid concentrations of sulbactam and ampicillin are obtained when these agents are administered parenterally and orally [8]. skin and soft tissue infections [5-7]. Sufficient penetration in peritoneal fluid. in resistance vitro activity.2. It contains esterified ampicillin and sulbactam and is marketed under a number of trade names. an interest for the oral administration of the combination has been developed for milder cases surveyed in several clinical studies. both formulations have similar efficacy as shown by comparable bioavailability of ampicillin and sulbactam (administered as oral sultamicillin) when equivalent quantities of the individual components are given intravenously [10. High tissue/fluid concentrations. sultamicillin [10.Ampicillin-sulbactam Expert Opin.2. As it is obvious for the treatment of severe infections. However. Drug Metab. Hampel et al.952) is an oral form of the antibiotic combination ampicillin/sulbactam [9]. Ampicillin/sulbactam is a β-lactam/β-lactamase-inhibitor combination that was first developed and marketed in US in 1987.14].4.v. Sulbactam sodium is a derivative of the basic penicillin nucleus.7-trioxo-4λ6-thia-1-azabicyclo[3. Toxicol. including upper and lower respiratory tract infections. Ampicillin has no effect on 1100 Either agent given alone has limited oral bioavailability.v. and for infants of < 30 kg 25 – 50 mg/kg in two divided doses [12]. the peak serum concentrations of ampicillin were twice as great for the prodrug [10]. our knowledge on the effectiveness in the treatment of community and hospital-acquired infections comes mainly from the use of its parenteral form applied in severe infections. 3. which is readily absorbed orally and hydrolyzed by enzymes in the intestinal wall. intestinal mucosa. urinary tract infections. these dosages have been exceeded [13]. no interaction in between occurs and both have similar profile of elimination half-time (∼ 1 h). Downloaded from informahealthcare. Expert Opin. releasing equimolar proportions of sulbactam and ampicillin to provide effective antibacterial activity [10]. in particularly severe infections.) and intramuscular administration (Figure 1).com by HINARI on 10/08/10 For personal use only. 2. which supports a 6 – 8 h i. the main body of evidence relies on information coming from studies with the parenteral form of the combination. and accumulation has not occurred in short-term multiple-dose trials [12].0] heptane-2-carbonyl]oxymethyl (2R)-{[(2S)2amino-2-phenyl-acetyl] amino}-3.3-di methyl-7-oxo-4-thia-1-azabicyclo [3. The pharmacokinetic profiles of ampicillin and sulbactam are similar and favor their co-administration either parenterally or orally (Tables 1 and 2).0]heptane2-carboxylate and has a chemical formulation C25H30N4O9S2 (Figure 1). Similarly. intra-abdominal infections. sulbactam is sodium penicillinate sulfone and its chemical formulation is C8H10NNaO5S [8]. Sultamicillin dosage for adults and infants of > 30 kg is 375 – 750 mg every 12 h. Steadystate volumes of distribution have been shown at the level of 0.
2 120 ± 16 281 ± 34 144 ± 64 Sulbactam (1 g) 42 ± 7 30 ± 10 1 ± 0.7 *Oral sultamicillin (750 mg) equivalent to 440 mg ampicillin and 294 mg sulbactam.69 66.4 ± 7.92 ± 0. ampicillin and sulbactam. As a result.d.11 ± 0. a dosing schedule of 6 – 8 h is indicated for the parenteral route [8] and a twice-a-day oral dosing schedule of sultamicillin is sufficient to achieve high serum levels of ampicillin and sulbactam into the circulation [10].com by HINARI on 10/08/10 For personal use only. the frequency of dosing is reduced according to creatinine clearance in 1101 Expert Opin. the tissue/fluid concentrations attained were generally in excess of the MICs for common pathogens associated with infections at these sites. Drug Metab. Co-administration of probenecid results in an increase in the half-lifes of both ampicillin and sulbactam.30 17. Chemical structures of sultamicillin.9 ± 3. Toxicol.29 16. demonstrating that tubular secretion is a significant component of their renal excretion [18]. Again.5 1. They are eliminated primary by urinary excretion: ∼ 65% of ampicillin and 46% of sulbactam from sultamicillin are recovered intact in the urine [11]. while biliary excretion is minor [8].2 0. the t1/2 and serum concentration in patients with renal impairment are increased [11].1 Sulbactam 8.8 ± 0. sputum and peritonsillar abscess pus has also been shown [11]. Table 1. Downloaded from informahealthcare. Half-lifes of both agents are similar and approximately equal to 1 h. Parameter Cmax (μg/ml) tmax (h) AUC (μg/(h ml)) t1/2 (h) Urinary recovery (% of dose) Ampicillin 9. Pharmacokinetic properties of intravenous ampicillin-sulbactam in healthy adults [14].7 1 ± 2. Esterase Ampicillin O S S N NH2 O N COOH O O HO H CHCONH H H O Sulbactam Figure 1. Based on half-life.96 ± 0. 3.2 72 ± 9 236 ± 27 136 ± 58 prostatic and appendiceal tissue. Parameter Cmax (μg/ml) Vds (l/kg) t1/2 AUC (μg/(h ml)) Clearance (ml/min) Renal clearance (ml/min) Ampicillin (2 g) 82 ± 15 31 ± 9 1 ± 0. Table 2.4 0.1 ± 3.) for ampiciln and sulbactam administered as sultamicillin* to healthy volunteers [85]. Toxicol.25 59. Given that both agents are primarily eliminated by renal excretion. (2009) 5(9) . good tissue penetration of sultamicillin supports its use in a twice-daily dosing regimen [10].Betrosian & Douzinas Sultamicillin O S O N NH2 C H O O S CONH N C O O CH2 O C O Expert Opin. The high bioavailability of sultamicillin assures that these high tissue concentrations are achievable with oral therapy [10]. Pharmacokinetic parameters (mean ± s.2 Metabolism and elimination Vds: Volume of distribution at steady-state.3 ± 6.96 ± 0.6 0. Drug Metab. Furthermore. Tubular secretion plays a major role in excretion of sulbactam.
Drug Metab. These resistant isolates usually evade the action of β-lactams by producing one of two β-lactamases [34]. 7. the resistance to β-lactam antibiotics of an organism is brought about by a complex interaction between the permeability of the bacterial cell to the antibiotic. fragilis isolates were resistant to the Expert Opin. During the last decade. the presence.7% of the B. Staphylococcus aureus. S.7%. resistant isolates among β-lactamase inhibitors have been developed and their in vitro and clinical efficacies compromised. Oral administration of a single dose of sultamicillin (42. However.134 aerobic and facultative Gram-negative bacilli [25].36]. 3. is particularly dependent on the time (T) that free serum concentration of the drugs exceed the MIC for the target pathogen (T > MIC) [21]. Gram-negative (including Haemophilus influenza. higher middle ear concentrations of ampicillin. pneumoniae was observed [33]. are other ways of resistance [32]. It should be noted that most β-lactamase inhibitors lack significant antimicrobial activity.6 to 19. a T > MIC of 30 – 40% of the dosing interval is required for maximal bacteriological efficacy against respiratory pathogens. 2001.6%. 3. Toxicol. 20.5 In vitro antimicrobial activity Early studies have shown a good in vitro activity of ampicillin-sulbactam in a wide range of Gram-positive (e.g. The postantibiotic effect (PAE) or persistent suppression of bacterial growth after brief exposure of a bacterial culture to an antimicrobial agent is a well-established phenomenon [23]. Using data from Alexander Project. level of production of β-lactamases. which hydrolyze the β-lactamic ring by forming of an acidic derivative of β-lactam deprived of antibacterial activity. Specifically.19]. Although the principal mechanism of aminopenicillin resistance observed in H.Ampicillin-sulbactam patients with renal impairment (from three or four times daily to twice or once daily). in children with otitis media. and supplemental doses are recommended after dialysis [8. the prevalence of penicillin resistance in S. 35. Streptococcus enterococci).com by HINARI on 10/08/10 For personal use only. The mechanisms involved in and responsible for resistance are several [31. Most β-lactams agents. Hemodialysis removes 30% of the given doses of ampicillin-sulbactam. the primary mechanism of resistance to this drug is mediated by changes in PBP [29.. Bacteroides spp. The drug is not active against Pseudomonas aeruginosa [25]. Regarding Gram-positive bacteria. In children. (2009) 5(9) . range of activity.30]. the main mechanism of resistance to ampicillin involves inactivation by β-lactamases.6 mg/kg sulbactam) resulted in greater peak serum concentrations and AUC of ampicillin than those achieved with equivalent dose of ampicillin alone in children whose weights ranged from 8. (73% susceptible). 3. Klebsiella pneumoniae. in a multi-center study with a total of 3.5 mg/kg of sultamicillin equivalent to 25 mg/kg ampicillin and 16. a more frequent administration scheme (every 4 h) is more appropriate due to a decreased elimination half-life [11]. particularly in Japan. with the greatest increase seen in France (1992. Escherichia coli.4 Pharmacodynamics Antibiotic pharmacodynamics describes the impact of an antimicrobial agent on a target pathogen and is based on the drug’s pharmacokinetics and microbiologic activity toward that pathogen. an 8-year period national survey in the US revealed that only 1. 5. Over the past decades. fragilis and other species of the Bacteroides group is increasing worldwide [35. Additionally. age has no effect on the pharmacokinetics of ampicillin or sulbactam and the results were also independent of dose and gender [12].6 Mechanism of resistance Expert Opin. pneumoniae increased between 1992 and 2001. For ampicillinsulbactam. influenzae is β-lactamase production.30]. Toxicol. over this 10 year period. the presence and efficiency of efflux mechanisms. For Gram-negative bacteria. Acinetobacter baumannii) and anaerobic bacterial pathogens (Bacteroides fragilis. Overproduction of β-lactamases is due to either a point mutation in the promoters for the genes encoding TEM-1 and TEM-2 or to multicopy. pneumoniae. Neisseriae (meningitides and gonorrhoeae) and Acinetobacter spp. Moraxella catarrhalis. an overall average increase in penicillin resistance in S. In Europe. including ampicillin. However.3 Pharmacokinetics in pediatric patients and Klebsiella spp. produce a short PAE when tested against Gram-positive cocci [24]. recent trials have indicated increasing resistance to the drug [25]. except for sulbactam which is active against Bacteroides fragilis. the incidence of infections caused by penicillin-resistant pneumococci has increased worldwide. and the affinity of the antibiotic to the penicillin-binding protein (PBP) target site [29.9 kg [20].8%) and the US (1992. sultamicillin produced prolonged PAE (about 3 days) for exposures of 50 – 100 μg/ml for > 2 h [23]. 2001. Ampicillin-sulbactam was found to be the least active agent among 12 antibiotics tested against E. were reported than after equivalent dose of ampicillin alone. Most bacteria with depressed class C β-lactamases are resistant to inhibitor combinations including ampicillin-sulbactam. and as such of ampicillin-sulbactam. administered as sultamicillin. such as inhibitor-resistant TEM and the OXA enzymes. coli (56% susceptible) 1102 In general. a number of β-lactamase-negative ampicillin-resistance strains have emerged is some countries. The rate of resistance to ampicillin-sulbactam among anaerobic pathogens such as B. The bacteriological efficacy of β-lactams agents. including Streptococcus pneumoniae [22]. Drug Metab. together with the pathogen’s susceptibility to the drug.32].4%) [33]. Overproduction of the class A penicillinases TEM-1 and TEM-2 and production of low affinity enzymes. [26-28].) [5]. In women during pregnancy. Pharmacokinetic properties of ampicillin-sulbactam are comparable in children and adults [12]. plasmid-borne genes [31]. Downloaded from informahealthcare. 3.
ampicillin-sulbactam has been used for prophylaxis in abdominal surgery [40]. endometritis. with the objective of producing a helpful prescribing tool. Over the past decades.v.5 – 3 g/day). Ampicillin-sulbactam should not be used in ESBL-producing bacteria regardless of an in vitro susceptibility being documented. organisms become highly effective at inactivating most β-lactam antibiotics. moderate diabetic foot infections and for the treatment of mild-tomoderate community-acquired complicated intra-abdominal infections [48-50]. IDSA and American Thoracic Society recommend the drug in combination with azithromycin or a fluoroquinolone as first-line treatment of patients with community-acquired pneumonia (CAP) admitted to a hospital’s intensive care unit and as a second-line treatment of CAP due to infection with Enterobacteriacae or Acinetobacter spp. Toxicol. skin and soft tissue infections. Escherichia coli is the most common agent from the enteric flora to cause UTIs. American Thoracic Society/IDSA guidelines recommend ampicillinsulbactam as first-line treatment of ‘hospital-acquired pneumonia’ or ventilator-associated pneumonia in patients with no known risk factors for multi-drug resistance (MDR) pathogens. except carbapenems [39]. a relative paucity of studies referring to sultamicillin exclusively is currently observed in the literature [45]. therefore.e.5 and 100%. IDSA guidelines support the use of ampicillin-sulbactam as an option in the initial treatment of necrotizing skin. This fact. It is. The best of these guidelines are evidence-based. The drug is currently recommended from the Infectious Diseases Society of America (IDSA) intra-abdominal infection guidelines as first-line agent for mild-to-moderate severity infections [37]. Extended-spectrum β-lactamases (ESBLs) are extremely broad spectrum β-lactamase enzymes found in a variety of Enterobacteriaceae [38]. tested the efficacy of a 3-day ampicillin-sulbactam prophylaxis scheme (3 g every 6 h) for the prevention of early-onset pneumonia in comatose mechanically ventilated patients. Downloaded from informahealthcare. professional organizations and societies from many countries have developed guidelines for the treatment of infections. Sultamicillin has not been evaluated in the setting of nosocomial infections caused by Acinetobacter. and sexually transmitted diseases [46-52].Betrosian & Douzinas Expert Opin. 4. acute or chronic sinusitis. Oral administration of ampicillinsulbactam was found to induce resistance (before treatment. The efficacy and tolerability of sultamicillin in these infections has been extensively evaluated in several comparative and non-comparative studies mainly in pediatric population in the past decades [7]. respectively [60-70]. For URTIs. Prophylaxis with ampicillin-sulbactam resulted in 64% reduction of the relative risk for early-onset pneumonia in the studied population [41]. infections following animal or human bites. is of potential interest for the management of urinary tract infections (UTIs). Perioperatively. other Klebsiella species (i. Despite the lower incidence of diarrhea observed with sultamicillin compared with amoxicillin/glavulanic acid. and supported by expert opinion. Drug Metab. respectively) both in adult and pediatric patients (Tables 3 and 4). ampicillinsulbactam (at i. bacterial septicemia. Neisseria gonorrhoea and anaerobes. fascia and muscle mixed infections. cholecystitis. Clinical trials Ampicillin-sulbactam has been used either parenterally or orally in a wide range of infections. and in several mixed infections including intra-abdominal infections such as peritonitis. early onset and any disease severity [47]. (2009) 5(9) . after treatment. intra-abdominal. or as a step-down therapy for patients who have improved under parenteral therapy [42-45]. brain abscesses. followed by oral sultamicillin in some cases) and oral sultamicillin (1. bone and soft tissue infections. Parenteral use is recommended in cases of community and/or hospital acquired pneumonia. Recent 1103 Expert Opin. including aspiration pneumonia. Resistance of the bacterial flora of the host is known to be induced after the prescription of antibiotics. In that order. Ampicillin-sulbactam has been recommended in nationwide guidelines as monotherapy or in combination with other antibacterials for various respiratory. respectively) [37]. coli. Drug Metab. Most strains producing these β-lactamases are K. influenza [51]. were adequate to achieve clinical success rates of 64 – 100% and bacteriological eradication rates of 68 – 100% [53-59]. When producing these enzymes. acute otitis media and periorbital cellulites. [46]. US Centers for Disease Control and Prevention recommend the drug as an option in combination with doxycicline as a treatment effective against Clamydia trachomatis. oxytoca) and E. ampicillin-sulbactam combination [36]. unsafe to draw clinical conclusions by extrapolating data of parenteral use. especially regarding the enteric flora. and for patients with tubo-ovarian abscess [52]. clinical success rates for LRTIs were in the range of 60 – 100% and 56 – 100%. European Respiratory Society and European Society of Clinical Microbiology and Infectious Diseases guidelines recommend aminopenicillin/β-lactamase inhibitor (± macrolide) as preferred treatment option for hospitalized patients with CAP and for treatment of ampicillin-resistant H. Klebsiella.1 Efficacy on respiratory tract infections Numerous studies in the late 1980s and early 1990s demonstrated the efficacy of ampicillin/sulbactam and sultamicillin in the treatment of upper and lower respiratory tract infections (URTIs and LRTIs. incisional surgical site infection after intestinal. axillary and genital tract surgery. and pediatric infections such as acute epiglotitis. doses of 2 – 6 g/day. The oral use of sultamicillin is indicated in less severe infections such as acute exacerbations of chronic bronchitis. 21%.5%) at a lower rate compared to ampicillin alone (87. with recommendations made only after extensive review and grading of studies in the literature. 37.com by HINARI on 10/08/10 For personal use only. because sulbactam dosages are quite different between the two formulations. Toxicol. perineum. otitis media. UTIs and cellulitis. 4. Acquarolo et al. pneumoniae..
b.i.d.d. 1104 Treatment Clinical efficacy (patients) Bacteriological eradication SM 0. p.d. b. Toxicol.o. otitis. b. p.o.5 g/d b. 10 days Amp/Sulb 1. [53] Tonsillitis. [112] AOM Amp/Sulb: Ampicillin/sulbactam. (2009) 5(9) Rodriguez et al. b. SM: Sultamicillin. Drug Metab. p. [57] AOM Expert Opin. p. or t. p.5 g b. pharyngitis Children Kaleida et al.d. [56] Acute sinusitis Ferreira et al. 10 – 11 days ND ND ND ND ND 49/49 (100%) isolates 32/44 (74%) patients 23/23 (100%) isolates ND Table 3.d. . p.5 b.: Three times daily.. Summary of studies examining efficacy of Amp/Sulb or SM in adult and pediatric patients with URTIs. URTI: Upper respiratory tract infection.i. peritonsilar abscess Nicoletti et al.o.d.o. ≥ 5 days SM 375 mg b.d. [45] Acute sinucitis. 10 – 11 days SM 50 mg/(kg day).d.d.i.. AOM: Acute otitis media.o. pharyngitis.o. ≥ 7days SM 25 mg/(kg day)..i. b.Expert Opin.o. cure at end of evaluation (97%) Relief of symptoms: 41/50 (84%) End of treatment: 47/51 (92%) Follow-up: 36/46 (78%) Cure 93/100 (93%) Improvement 6/100 (6%) Cure 42/49 (86%) Improvement 7/49 (14%) Cure 17/26 (65%) Improvement 9/26 (35%) Effusion-free: end of treatment 43/81 (53%).d.i.o. Toxicol.. p.i. 10 days.. evaluation at 30 days SM 50 mg/(kg day) (b. 5 – 7 days SM 375 mg b. p.i.d.i. IM 5 – 10 days AOM: cure 12/19 (63%) Improvement 5/19 (26%) Acute/chronic sinucitis: cure 12/22 (55%) Improvement 10/22 (45%) Cure 46/55 (84%) Improvement 9/55 (16%) Improvement + cure: 28 + 21/66 (74%) Cure at end of treatment (64%).i.d. [54] Acute AOM or Acute/ chronic sinusitis Alvart [55] Acute ear-nose-throat infection (mild/moderate) Topuz et al. ND: No data.com by HINARI on 10/08/10 For personal use only. Drug Metab. Downloaded from informahealthcare.o. or t.o. [59] Mild-to-moderate URTIs Arguello [110] Mild-to-moderate URTIs Biolcati [111] AOM Chan et al.75 or 1.i.d.). Ampicillin-sulbactam Study Site of infection Adults Federspil et al. [58] AOM Raillard et al.o. follow-up 48/84 (57%) Cure 55/55 (100%) Follow-up 40/42 (95%) 24/29 (83%) isolates 17/25 (68%) isolates 22/22 (100%) for acute/ chronic sinucitis SM 0.i.i. t. 10 days SM 25 mg/(kg day).i. p. ≥ 7 days SM 50 mg/(kg day).i.: Twice daily..d.. p.). p.d.i.: Oral. 10 days SM 50 mg/(kg day) or 500 mg/day (b.
[62] Schwigon et al.5 3 g q 12h IMP/CIL 0. Toxicol.5 g/12h 1.i. Amp/Sulb: Ampicillin/sulbactam.6 q8h IMP/CIL 1. CLI: Clindamycin.d.v.9 84 90.6 g q12h 3 g q8h CLI ± CEPH 0.v. [68] Betrosian et al. MOX: Moxifloxacin. Summary of comparative studies examining efficacy of Amp/Sulb or SM in adult patients with LRTIs. NR: Not reported. Toxicol.i.i.5 g q8h 31/35 (89%) 3 g q6h CFT 2 g q6h 29/34 (85%) 1.i. p.75 g b.com by HINARI on 10/08/10 For personal use only.5 82 88. COL 3MIU q8h 9/15 (60%) 9/13 (61.d.d. q. CFX 0.Expert Opin. IMP/CIL: Imipenem/cilastatin. b.75-1.6 61.: Twice daily.i. p.: Intravenous. 19/19 (100%) 17/18 (96%) 13/16 (81%) 28/35 (80%) 38/46 (83%) (78%) 52/63 (83%) 22/33 (67%) 19/25 (76%) 22/25 (88%) 28/32 (88%) 32/48 (67%) Comparator (i.d. p. [70] AECB: Acute exacerbation of chronic bronchitis. i.v. Infection Amp/Sulb (i. MOX 0.5 – 3 g q. VAP: Ventilator-associated pneumonia. (2009) 5(9) Kadowaki et al. Drug Metab. Drug Metab.4 g.: Oral. PAPM/BP: Panipenem/betamiprom.o. [65] Expert Opin. 1105 . t. LRTI: Lower respiratory tract infection.5 g/12h 3 g q12h PAPM/BP 0. CFT: Cefoxitin. 3 g q8h i. TIC/CLA: Ticarcillin/clavulanic acid. COL: Colistin.) Pneumonia AECB LRTIs LRTIs Pneumonia AECB LRTIs VAP Aspiration Pneumonia Aspiration Pneumonia Pneumonia Aspiration Pneumonia VAP 9 g q8h 0. [67] Ott et al.4 g/d 0.i.o. Four times daily. SM: Sultamicillin. CEPH: Cephalosporin. CFX: Cefuroxime.5 t.: Three times daily.d. Downloaded from informahealthcare.d. Study Geckler [60] Jauregui et al. MIU: Million units.5 – 3 g q4h TIC/CLA 3. [66] Yanagihara et al.5%) 66. MZL: Mezlocillin.v.1 g q6h (79%) 13/14 (93%) 27/37 (73%) 19/25 (76%) 21/25 (84%) 32/35 (91%) 32/48 (67%) 3 g q8h MZL 4 g q8h 42/50 (84%) 3 g q8h CFX 1.) Amp/Sulb Comparator Antibiotics Clinical efficacy (pts %) Bacteriological eradication (%) Amp/Sulb 100 55..2 60 100 NR 72 68 84 NR 76 80 80 Comparator 94 62. [69] Allewelt et al. [63] McKinnon and Neuhauser [64] Wood et al.5 g q12h CLI 0.9 71 97 Betrosian & Douzinas Table 4.o. [61] Schwigon et al.
Likewise.. Patients were randomized to receive either 3 g ampicillin-sulbactam or 2 g of cefoxitin every 6 h. the initial choice of antimicrobial therapy on the length of stay for patients with complicated intra-abdominal infections was assessed. 4. t.9% of the comparator agent.69-71]. Several early studies have established the antimicrobial usefulness of ampicillin-sulbactam in patients with intraabdominal infections [6. aspiration pneumonia.Ampicillin-sulbactam comparative studies confirm previous findings for both URTIs and LRTIs [45. In fact. vomiting. such as E. Lower UTIs are very common in infancy and childhood. [73] compared the efficacy and safety of ampicillin-sulbactam with cefoxitin in a double-blind trial in patients with suspected bacterial intra-abdominal infections. in a randomized. lung abscess and ventilator-associated pneumonia (Tables 3 and 4). including CAP.001) to those achieved by ampicillinsulbactam [64].56. Furthermore. reported equal effectiveness (cure or improvement) of ampicillin-sulbactam (2/1 g q6h) versus cefoxitin (2 g q6h) regarding clinical and bacteriological success.3 UTIs Expert Opin. Talan et al. in a recent non-comparative study reported 100% clinical (cure plus improvement) rates in six patients with UTIs treated with a median 9 g/day of ampicillin-sulbactam [28]. A review of randomized-controlled trials examining patients with intra-abdominal infections and/or peritonitis showed that sultamicillin had comparable clinical success rates when compared with all antibiotic regimens (cefoxitin. results from comparative studies demonstrated equivalent clinical efficacy to comparator agents (81 – 95% versus Expert Opin. Drug Metab. the comprehensive reviews by Dajani [77] and Kanra [78] showed that ampicillinsulbactam was as effective as and in some cases superior to the antimicrobials traditionally used for the treatment (including surgical prophylaxis) of intra-abdominal infections [79]. 4. ampicillin-sulbactam (at i. Other studies demonstrated that ampicillin-sulbactam (doses from 4 to 12 g/day) had comparable efficacy to clindamycin.5 Gynecological/obstetrical infections Numerous comparative and non-comparative studies in the past decades demonstrated the efficacy of ampicillinsulbactam (including sultamicillin in some cases) in the treatment of obstetric and gynecological infections. those with absence of microbial documentation of the infection and those with early interruption of treatment. ticarcillin/clavulanic) with the exception of one (clindamycin plus gentamycin). imipenem/cilastatin and piperacillin/ tazobactam.) and concluded that administration of sultamicillin was advantageous due to simplest daily scheme [81].87].4 g/day) [64]. diarrhoea and rush [73]. bacteriological eradication rates were comparable in all studies providing relevant data except for one. Although ampicillin-sulbactam is not considered the first-line treatment option for the so-called pelvic inflammatory disease. Specifically. 4. gentamycin/ ampicillin. Toxicol.com by HINARI on 10/08/10 For personal use only. ceftriaxone and piperacillin/tazobactam [76]. Recently. The report from the study group of intraabdominal infections showed that ampicillin-sulbactam had comparable efficacy rates (cure plus improvement) with gentamicin plus clindamycin (87 versus 97%.d. ticarcillin/clavulanic. Bacterial eradication was achieved in 100% of patients receiving ampicillin-sulbactam versus 97. imipenem/cilastatin. Levin et al. 1106 Several studies in the past decades found that treatment with ampicillin/sulbactam or sultamicillin was effective for various UTIs [80-82].72. Walker et al. One hundred ninety-seven patients were evaluable for clinical efficacy excluding not operated patients.v. comparative bacteriological eradication rates (83 versus 66%) and concluded that no difference between the two antibiotic regimens existed [74]. ampicillinsulbactam provided clinical response comparable with imipenem/cilastatin (81% (n = 48) versus 85% (n = 48)) [86]. with clinical efficacy > 80% and eradication rates of 41 – 100% [64. Drug Metab. Ferreira et al. in a multi-center study comprising 2150 patients. which showed significantly higher efficacy rates than ampicillin-sulbactam in the treatment of gangrenous and perforated appendicitis [72. As referred earlier.2 Efficacy on intra-abdominal infections With regard to pediatric population. double-blind comparative trial. clindamycin and colistin for the treatment of LRTIs. (2009) 5(9) .75]. doses 3 – 18 g/day. Ampicillinsulbactam demonstrated clinical success of 86% (n = 96) versus 78% of cefoxitine (n = 101) with no differences in the nature or frequency of side effects observed in the two groups. The predominantly renal route of excretion for ampicillinsulbactam and high urinary concentrations favor the eradication of common urinary pathogens. In the treatment of limbthreatening infections in diabetic patients.73].4 Soft-tissue infections Ampicillin-sulbactam has been considered a useful agentcombination in treating various soft skin tissue infections including diabetic foot [83-85]. 4. Similar cure and eradication rates were also found in a study comparing ampicillin-sulbactam (6 – 12 g/day) and ticarcillin/clavulanic (12. Downloaded from informahealthcare. and rarely up to 36 g/day) followed by oral sultamicillin in some studies is comparable with moxifloxacin. coli [77].86. only 30% of patients in each group presented mild symptoms such as nausea. reported comparable efficacy rates for oral sultamicillin (750 mg daily) when compared with 1.5 g/day of amoxicillin/clavulanic for the treatment of common community URTIs [45]. in which the eradication rates achieved with ticarcillin/clavulanic were significantly inferior (p = 0. in patients with or without a history of injection drug abuse who presented with cutaneous or other soft tissue infections [85]. Schutz showed that sultamicillin (750 mg twice daily) had similar clinical efficacy when compared to amoxicillin/clavulanic (625 mg thrice daily.i. respectively). Mean length of stay analyzed by initial antimicrobial therapy and stratified by diagnosis showed to be significantly shorter for sultamicillin compared with levofloxacin. Toxicol.
1107 Expert Opin. Favorable clinical outcomes have been reported with ampicillin-sulbactam therapy in patients with various types of nosocomial infections caused by MDR A. 3 g in 38 patients in group 2) versus ticarcillin/clavulanic acid (5 patients in group 3) had comparable clinical (overall clinical efficacy 93 versus 100%. respectively). showed improvement in 9 (90%) of 10 patients with imipenem-resistant Acinetobacter calcoaceticus infections treated with ampicillin-sulbactam. [102] evaluated ampicillin-sulbactam (2 g/1 g t.i. Betrosian et al. Urban et al. Corbella et al. baumannii isolates [100. the cost effectiveness was significantly lower in the ampicillin-sulbactam group than in the ticarcillin/clavulanic acid group [64]. non-spore forming.7%. Similarly. sultamicillin seemed to be more efficacious therapy than polymixins. baumannii. respectively) and bacteriological efficacy rates. Toxicol. cefotetan. meningitis and UTIs. baumannii resistance to this agent in patients with ventilator-associated pneumonia [13]. 81% of which were genetically related. 86% were treated according to susceptibility and 14% were treated inappropriately with antibacterials to which these organisms were resistant. baumannii bloodstream infections. and end of treatment and was a cost-effective alternative for treatment of A. data from several studies have showed that lower doses of the drug can be effective against MDR A. ampicillin-sulbactam therapy significantly decreased the risk of death (p = 0. in a retrospective study showed that ampicillin-sulbactam was at least as effective as imipenem-cilastatin based on clinical response at days 2. whereas of 43 patients with non-MDR A. Notably.8%. bactaeremia. 139/153) against postpartum endometritis infection [89]. Acinetobacter baumannii is a Gram-negative. throat and other sites of healthy persons.v. Oliveira et al. Of the 51 patients with MDR A.5 g in 24 patients in group 1.) in the treatment of non-life threatening MDR strains and showed that the active drug against A. baumannii. MDR is a growing problem and has been reported in different parts of the world. baumannii strains.) compared with sulbactam alone (1 g t.104]. the Centers for Disease Control and Prevention guidelines suggest ampicillinsulbactam plus doxycycline as one of the alternatives to treat pelvic inflammatory disease [92]. baumannii.5% of clinical success rate (improvement/care) when treated with 3 – 18 g i.Betrosian & Douzinas Expert Opin.02. Sulbactam has intrinsic antimicrobial activity against A. including cefoxitin. metronidazole/gentamycin and clindamycin alone [88-91]. However.96]. non-fermentative. However. (2009) 5(9) . Drug Metab. Toxicol. sulbactam retains its activity against a rather small proportion of carbapenem resistant A.4 and 91. and is the most active of the β-lactamase inhibitors [3. 83 – 95%). [70] in a small prospective trial using high dosing scheme of ampicillin-sulbactam showed comparable efficacy and safety between the two drugs for treatment of critically ill patients with MDR A. However.com by HINARI on 10/08/10 For personal use only. particularly in the intensive care setting [95].5%. bloodstream infections. Cisneros et al. causing significant difficulties in the treatment of these severe hospital infections. baumannii. Studies from the past decades have shown high in vitro susceptibility (using the National Committee for Clinical Laboratory Standards criteria) rates of A. 65% received ampicillin-sulbactam and 35% received inadequate antibacterial therapy. Among severely ill patients. to ampicillin-sulbactam [97-99]. oxidase-negative coccobacillary organism that may be isolated from skin. which was an independent factor associated with mortality during treatment. Acinetobacter baumannii is a major cause of hospital infections including pneumonia. baumannii isolates. [98] treated 8 patients with ampicillin-sulbactam and 42 with imipenem. ampicillin-sulbactam showed comparable efficacy with clindamycin/gentamicin (88. Drug Metab. Levin et al. of ampicillin-sulbactam (median 9 g/daily). meningitis and UTIs [94]. baumannii isolates has declined substantially. In a study of 94 patients with nosocomial A. high-dose ampicillin-sulbactam regimens have been used with safety as a means to overcome A. respectively. respectively. [97]. baumannii bacteremia. it has been shown that doxycycline plus cefoxitine had no advantage over ampicillin-sulbactam in the treatment of pelvic inflammatory disease and both regimens have equal clinical response of about 70% [93]. Its mode of activity is either bacteriostatic or bactericidal depending on the strain examined. including MDR ones. and is mediated through binding to PBP. Still. Moreover. Downloaded from informahealthcare. cefoxitin/doxycycline. 54% involved MDR A. baumannii infections [104]. Wood et al.i. baumannii. Contrariwise. Infections due to Acinetobacter spp. Respective mortalities among patients treated adequately and inadequately were 41. [103] in a retrospective comparative study of 75 patients with ventilatorassociated pneumonia showed similar treatment efficacy in the ampicillin-sulbactam and imipenem-cilastatin groups (93 versus 83%.64) [105].7% (p < 0. odds ratio = 7. In a prospective observational cohort study of patients with A.001). A retrospective study involving gynecological infections showed that ampicillin-sulbactam (1. the antimicrobial activity of sulbactam against A. and observed response rates of 83. However.5. baumannii ventilator-associated pneumonia. Crude mortality was comparable in the adequately treated groups. strictly aerobic. Baumannii as well [101. baumannii. Two late comparative studies evaluated the efficacy and safety of ampicillin-sulbactam versus polymixins (colistin) for treating infections caused by MDR A. including ventilator-associated pneumonia. in recent studies.0 and 87. gentamycin/clindamycin. baumannii in the ampicillin-sulbactam combination is sulbactam. Specifically. In a non-comparative study of 40 consecutive patients with severe nosocomial infections by MDR A. Jellison et al. 141/159) versus (90.d. [101] recorded 67.101]. 7. [106] retrospectively showed that among existing therapeutic options for infections due to carbapenemresistant Acinetobacter.d. 5.
The drug is effective for the treatment of URTIs and LRTIs. Because animal reproduction studies are not always predictive of human response. or for disease caused by single or several susceptible bacteria in both adults and children with a broad range of community and hospital acquired infections. aeruginosa and pathogens producing ESBLs and. Ampicillin-sulbactam is generally well tolerated and its oral form. allergic response or sensitization) and interference with the interpretation of culture results if a fever workup is required. Toxicol. Animal studies have failed to reveal evidence of fetal harm. Safety and tolerability 7.45]. provides effective outpatient therapy against many commonly encountered infections. hospital acquired infections should not be treated with ampicillin-sulbactam empirically and treatment should be adapted on the basis of pathogen identification. especially during intramuscular administration. diabetic foot and skin and soft tissue infections. pseudomonas and ESBL-producing bacteria in the nosocomial setting. is not recommended for infections due to these organisms. and found that the drug was well tolerated while no major adverse effects were reported [13]. no controlled data from human pregnancy studies. sexually transmitted infections. 8. Other symptoms included soft stools (2. in the era of increasing bacterial resistance. two case reports of cholestasis associated with the drug have been published in the recent literature [107]. Drug Metab. nausea (1. Almost 50% of these reactions involved pain at the injection site.2%. Data on the safety of ampicillinsulbactam collected from 1287 patients from a variety of studies demonstrated adverse reactions at 10% of patients involved compared with 6% of comparator treated patients [6]. with diarrhoea to be the most frequently reported adverse reaction with an overall incidence of 10. with the ratio of patients with diarrhoea to be significantly higher in the amoxicillin-clavulanic acid group.2%).com by HINARI on 10/08/10 For personal use only. improving Expert Opin. therefore. baumannii ventilatorassociated pneumonia. however. Expert opinion The discovery of the β-lactam inhibitor sulbactam and its co-administration with ampicillin as ampicillin/sulbactam allowed the continued use of this antibiotic to treat infections caused by pathogens that had developed resistance due to β-lactamase production. gynecological.4%). serum alanine aminotransferase. Other adverse reactions were skin disorders (1. this drug should be used during pregnancy only if clearly indicated [108].v.3%). Although adverse effects are apparently rare. direct effects on the infant (e. Discontinuation of treatment because of diarrhoea was observed in 2. Ampicillin-sulbactam is a β-lactam/β-lactamase inhibitor combination with a broad spectrum of antibacterial activity that includes Gram-positive and Gram-negative aerobic and anaerobic bacteria. thus. With regard to oral sultamicillin. Toxicol.8% of patients. Milk:plasma ratios have been reported up to 0.9% of patients. (2009) 5(9) . formulation. Ferreira et al. baumannii.9%). Numerous clinical trials and several meta-analyses have demonstrated ampicillin-sulbactam to be as clinically effective as relevant comparator antibiotics.4%. One of the specific advantages of using ampicillin/sulbactam combination when compared with other β-lactam/β-lactamase combinations is the inherent activity of sulbactam against A. Significant hepatotoxicity has not been reported with the use of ampicillin-sulbactam. ampicillin-sulbactam preserves its critical place in worldwide updated guidelines for the treatment of various infections including URTIs and LRTIs. Side effects were recorded in 17. Downloaded from informahealthcare. However. intra-abdominal infections.. a summary of data derived from 5947 patients with a variety of communityacquired infections has provided evidence for a satisfactory tolerability profile [7]. [45] reported similar adverse reactions in patients treated with ampicillin-sulbactam versus amoxicillin-clavulanic acid. Ampicillin-sulbactam has been assigned to pregnancy category B by the FDA. diarrhoea (1.) or sultamicillin have been well tolerated in clinical trials both in adults and children [6.g. Drug Metab. making the drug a valuable option against multi-resistant isolates. Recent studies confirm previous finding regarding safety and tolerability [13.6%) and minor increase in serum transaminase levels (serum aspartate aminotransferase. Ampicillin-sulbactam is not active against P. loose stools (1. Clostridiun difficile or its toxin was not observed in the stools from patients with diarrhoea in all except one who had previously experienced a similar episode with clindamycin therapy and had uneventful course following vancomycin therapy. Our group used high dosing schemes of ampicillin-sulbactam (27 – 36 g/day) for treating A. Its broad spectrum of activity against several β-lactamase-producing pathogens. The drug is indicated either parenterally (ampicillin/sulbactam) or orally (sultamicillin) as empirical therapy before the identification of causative organisms.v. thus expanding the range of activity of ampicillin alone. thus.9%) [6].7]. There are. 6. 1108 Expert Opin.1%) and rash (0. Furthermore. sultamicillin. Numerous studies and a number of meta-analyses demonstrated the usefulness of the combination of ampicillinsulbactam (administered parenterally or orally) for a number of clinical indications. 6. combined with a favorable safety and tolerability profile. three potential problems exist for the nursing infant: modification of bowel flora. the availability of oral sultamicillin also provides the option of oral follow-on therapy on an outpatient basis after the initiation of treatment with i.Ampicillin-sulbactam 6. makes this antibacterial a first-line option for the empirical treatment of mild to moderate community acquired infections. UTIs. Due to the high prevalence of MRSA.2 [109]. Ampicillin is excreted into breast milk in low concentrations. however. intra-abdominal and skin and soft-tissue infections. Conclusions Ampicillin-sulbactam (administered intramuscular or i.
Swanson RN. Antimicrob Agents Chemother 1987. Sex Transm Dis 1991. 14. Downloaded from informahealthcare. Frantzeskaki F. et al. McCracken GH Jr. 27. pharmacokinetic properties. Toxicol. Toxicol. 28. Drugs 1988. Time-kill kinetic studies of ampicillin/sulbactam for beta-lactamase-producing enterococci. The pharmacokinetics of sultamicillin. Morrison PJ.15:345-51 Patterson JE. McBride TJ. Clin Pharm 1992. Perez-Diaz JC.8(Suppl 5):S503-11 Nahata MC. Snyder TA. Cheong YM. Betrosian AP. Sulbactam/ampicillin. 26. 8. Sulbactam plus ampicillin: interim review of efficacy and safety for therapeutic and prophylactic use. FEMS Microbiol Rev 2008. Knirsch AK. 29. Johnson CA. The postantibiotic effect. Randhawa B. Brogden RN. Int J Antimicrob Agents 1999. Vernet T. Microbios 1999.105:395-424 Parker RH. APMIS Suppl 1989. Drugs 1987. et al. et al. Zervos MJ.27:569-75 Georgopapadakou NH. The effect of a combination of ampicillin and sulbactam against clinical isolates of anaerobic bacteria.Betrosian & Douzinas patients’ compliance. Lees LJ. 31. Clin Microbiol Infect 2002. 21. Rational antibiotic therapy and the position of ampicillin/sulbactam. Pharmacokinetics and bioavailability of sultamicillin estimated by high performance liquid chromatography. A review of its antibacterial activity.6(Suppl):S27-30 Ngeow YF. Eggleston M. Fisher JF. Penetration of ampicillin and sulbactam into human costal cartilage. Surg Infect (Larchmt) 2005. Stankewich JP. Ampicillin and sulbactam pharmacokinetics and pharmacodynamics in continuous ambulatory peritoneal dialysis (CAPD). discussion S26-27 Fu KP. 25. et al. et al. Bacterial resistance to beta-lactam antibiotics: compelling opportunism. Declaration of interest The authors state no conflict of interest and have received no payment in preparation of this manuscript. Milson JA. Cox DA. Expert Opin. Bradbrook ID. Baquero F. Comparative pharmacokinetics of sulbactam/ampicillin and clavulanic acid/ amoxycillin in human volunteers. Bruckner G.22:152-5 Emmerson AM. Rev Infect Dis 1986. 10. and activity in models of acute infection. Mobashery S. Contreras-Martel C.16:45-9 23. 12.9:1145-65 Zapun A. et al. Pharmacokinetics of sulbactam/ ampicillin in humans: a review.39:38-43 Foulds G. et al. 11. 17. 30. J Antimicrob Chemother 1983. et al. 4. Prabhakaran K. J Antimicrob Chemother 1985. Leggett JE. 9. J Antimicrob Chemother 1991. Eur J Clin Microbiol 1983. Sulbactam/ampicillin: in vitro spectrum. 19. et al. Diagn Microbiol Infect Dis 1991. Neu HC.8(Suppl 5):S644-50 Lode HM. Postantibiotic effect of ampicillin/ sulbactam against mycobacteria.10:221-6 Ginsburg CM. 5. 3. ventilator-associated pneumonia. Int J Antimicrob Agents 2008.5:17-22 Foulds G. Rev Infect Dis 1986. Bruckner G. English AR. Pharmacokinetics of sulbactam and ampicillin following oral administration of sultamicillin with probenecid.35(Suppl 7):29-33 Foulds G. Perit Dial Int 1990. Vashi VI. Satishchandran V. Pharmacokinetics of ampicillin and 22. 15.99:113-22 Spivey JM.11:865-75 Chow JW. Multiresistant Acinetobacter infections: a role for sulbactam combinations in overcoming an emerging worldwide problem. Beta-lactamase inhibitors: evolving compounds for evolving resistance targets. Comparative inhibition beta-lactamases by novel beta-lactam compounds. Treatment of gonorrhea with sulbactam/ ampicillin. et al. Springsklee M. 7. Penicillin-binding proteins and b-lactam resistance. Expert Opin Investig Drugs 2004. Eur Respir Rev 2007. compelling opportunity. Marshall DC. et al. Penetration of sulbactam and ampicillin into cerebrospinal fluid of infants and young children with meningitis. Infect Control 1987.32:10-28 Campoli-Richards DM. Girard A. Farrel P. Meroueh SO. Wildfeuer A.33:577-609 Hj RO. Factors determining resistance to beta-lactam combined with beta-lactamase inhibitors in Escherichia coli. Pharmacokinetics of sulbactam in humans. Antimicrob Agents Chemother 1999. Heilmann F. Chem Rev 2005. (2009) 5(9) 1109 .12(Suppl 1):S3-7.com by HINARI on 10/08/10 For personal use only. Rev Infect Dis 1986. et al. 32. Drug Metab.15:171-6 Retsema JA.31:1703-5 Meier H. Knirsch AK.13:1307-18 sulbactam in pediatric patients.23:692-9 Hampel B.43:1225-9 13.8:144-53 Sandanayaka VP. Antimicrob Agents Chemother 1979. 24.32:361-85 Reguera JA. Beta-lactamases and beta-lactamase inhibitors. Xanthaki A. Curr Med Chem 2002. Drug Metab. Int J Antimicrob Agents 1996.11:435-45 Lode H.8:36-40 Williams JD. and therapeutic use. In vitro susceptibilities of aerobic and facultative gram-negative bacilli isolated from patients with intra-abdominal infections worldwide: the 2002 Study for Monitoring Antimicrobial Resistance Trends (SMART). Prashad AS. Pharmacokinetic optimisation of ß-lactams for the treatment of 2. Beta-lactamase inhibitors: another approach to overcoming antimicrobial resistance.14:495-9 Drusano G. High-dose ampicillin-sulbactam as an alternative treatment of late-onset VAP from multidrug-resistant Acinetobacter baumannii. Harris EB. 20. Scand J Infect Dis 2007.8(Suppl 5):S528-34 Lees L. Heizmann W. Antimicrob Agents Chemother 1983. Ampicillin-sulbactam has been recommended in nationwide guidelines as first-line therapy or in combination with other antibacterials for various respiratory.18:192-4 Levin AS.6:439-48 Werner H. Resistance to beta-lactam antibiotics: structure and mechanism based design of beta-lactamase inhibitors. Hampel B. Bibliography 1. Lode H. Ramachandran S. intra-abdominal and skin and soft tissue infections. potency. Pharmacokinetics and bactericidal activity of sultamicillin in infants and children. Olsen K. 18. 6. Expert Opin.2:340-4 Blackwell BG. 16. Infection 1994. et al.
Huang YT.18:657-86 Wenzel M. Low dose sultamicillin in acute sinusitis. et al. et al. Pharmacokinetics of ampicillin/sulbactam in patients undergoing colorectal surgery: measurements in serum.10:45-8 Kaleida PH. Khan WH.39:885-910 Solomkin JS. Perone G.171:388-416 Stevens DL.19:724-33 Allewelt M. Weerasinghe HD. Ozkan H. Toxicol. et al.Ampicillin-sulbactam 33.20:368-89 Liu CY. 52.16:662-72 Jauregui L. 38. Eur R J 2005. Bolcskei PL.37:997-1005 Woodhead M. APMIS Suppl 1989. sultamicillin with that of cefuroxime axetil in acute ear nose and throat infections in adults. Berendt AR.6(Suppl):S73-7 Schwigon CD. 45. Clin Microbiol Rev 2005. 44. Chambers HF. Clin Microbiol Infect 2005. 62. Diagnosis and treatment of diabetic foot infections. Antimicrob Agents Chemother 2007. 55. Katircioglu O. et al. the colonic wall and in tissue at the incision site. 1110 Expert Opin. Sakano E. Cuhorst R.51:1649-55 Solomkin JS. 63. Downloaded from informahealthcare. 54. Drug Metab.55:RR-11 Federspil P. Braz J Otorhinolaryngol 2006. 40. Jacobus NV. Deery HG. 37. Mizuno S. McDermott LA.11(Suppl 4):1-16 Paterson DL. Pharmacotherapy 1999. 42.31:510-6 Chang ST. 41. Reappraisal of clindamycin IV 34. (2009) 5(9) . Clin Ther 1994. et al. multicentric. Ewing S. Bayramoglu I. 51. Diagn Microbiol Infect Dis 1989. and healthcare-associated pneumonia. Increasing trends in antimicrobial resistance among clinically important anaerobes and Bacteroides fragilis isolates causing nosocomial infections: emerging resistance to carbapenems.20(Suppl 1):12A-23A Geckler RW. Appelbaum PC. Clin Infect Dis 2007. Wildfeuer A. et al. J Chemother 1995. Jacobs MR. et al. Which antibiotic is better for the treatment of infections of the lower respiratory tract? Int J Antimicrob Agents 1996. randomized and comparative study.5:51-6 Airede AI. 56. Comparison of sulbactam/ampicillin and cefuroxime in infections of the lower respiratory tract: results of a prospective. et al.72:104-11 Mandell LA. Drug Metab. Gabor M.56(Suppl S2):ii3-21 Tristram S. et al. 48. Demura Y.com by HINARI on 10/08/10 For personal use only. A comparison of ampicillin/sulbactam versus cefotaxime in the therapy of lower respiratory tract infections in hospitalized patients. Speciale A. Bluestone CD.44(Suppl 2):S27-72 American Thoracic Society. 57. Sait T. Clin Microbiol Infect 2004. Antimicrob Agents Chemother 2008.5:45-50 Nicoletti G. Sulbactam/ampicillin followed by oral treatment with sultamicillin for medical and surgical infections. Sultamicillin (sulbactam/ampicillin) versus amoxycillin in the treatment of acute otitis media in children. Antimicrobial resistance in Haemophilus influenzae. et al. et al. Efficacy and cost of ampicillin-sulbactam and ticarcillin-clavulanate in the treatment of hospitalized patients with bacterial infections. A comparison of ampicillin/ sulbactam and cefuroxime in the treatment of patients with bacterial infections of the lower respiratory tract. 59. Wunderink RG. et al. Clin Infect Dis 2004. White AR. Anzueto A. Guidelines for the management of adult lower respiratory tract infections. Pai SD. 64. Pediatr Infect Dis 1986. 35. Urli T. et al. Kose G. Efficacy and safety of Sultamicillin (Ampicillin/Sulbactam) and Amoxicillin/ Clavulanic acid in the treatment of upper respiratory tract infections in adults–an open-label. An open multicentre study to compare the efficacy and safety of 66. 39. Am J Resp Crit Care Med 2005.6(Suppl):S67-72 McKinnon PS. Intensive Care Med 2005. Clin Infect Dis 2003. Chung HY. Caccamo F.10:163-70 Kadowaki M. 2006.41:1373-406 Lipsky BA. Int J Antimicrob Agents 1997. Blasi F.18(Suppl 4):78D-84D Raillard P. J Int Med Res 1992. et al.12(Suppl 4):S175S-8 43. randomized trial. Sultamicillin versus amoxicillin in the treatment of tonsillitis and pharyngitis: a European multicenter study. 50. et al. et al. A randomized study. Liao CH. Ampicillin + sulbactam vs clindamycin +/cephalosporin for the treatment of aspiration pneumonia and primary lung abscess. Bonomo RA. Int J Antimicrob Agents 1996.5:33-8 Rodriguez WJ.7:153-6 Schwigon CD. Neuhauser MM. 47. Jacobs MR. Antibiotic prophylaxis of early onset pneumonia in critically ill comatose patients. 53. et al. Hartmann J. Expert Opin. Mazuski JE. Guidelines for the selection of anti-infective agents for complicated intra-abdominal infections. Mazuski JE. et al. et al. et al. Jalo I. Low-dose sultamicillin oral suspension in the treatment of mild to moderate paediatric infections in Turkey. Clin Infect Dis 2005.52:3161-8 Snydman DR. et al. practice guidelines for the diagnosis and management of skin and soft tissue infections. 49.37:997-1005 Giamarellou H. Gutsche F. Toxicol. Guidelines for the management of adults with hospital-acquired. ventilator-associated. Bisno AL. 60. Schuler P. Sultamicillin (ampicillin-sulbactam) in the treatment of acute otitis media in children. et al. J Int Med Res 1990.26:1138-80 Centers for Disease Control and Prevention (CDC). 65. Gabor M. Felmingham D. 58. 61. Kobayashi H. J Int Med Res 1991. National survey on the susceptibility of Bacteroides fragilis group: report and analysis of trends in the United States from 1997 to 2004. APMIS Suppl 1989. Grunbacher G. Lejdeborn L. Observations on oral Sultamicillin/Unasyn CP-45 899 therapy of neonatal infections. Infez Med 2002. Blatter MM. The Alexander Project: the benefits from a decade of surveillance.20(Suppl 1):53A-61A Topuz B. Clin Microbiol Rev 2007. Rapoport PB. Minns P. Int J Antimicrob Agents 1996. MMWR 2006. Hageage G.6(Suppl):S35-9 Acquarolo A.8:103-7 Ferreira JB. 36. Sexually transmitted diseases treatment guidelines. Baron EJ. Baron EJ. Hara K. J Int Med Res 1992.19(Suppl 1):29A-35A Alvart R. Clin Infect Dis 2003. Sultamicillin experiences in the field of internal medicine. Guidelines for the selection of anti-infective agents for complicated intra-abdominal infections. Infectious Disease Society of America/ American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Multidrug resistance in Gram-negative bacteria that produce extended-spectrum beta-lactamases (ESBLs). Sulbactam/ampicillin in the treatment of otitis and sinusitis. 46. et al. J Antimicrob Chemother 2005. Extended-spectrum beta-lactamases: a clinical update.
Plummer FA. Hartman BJ.38:1449-59 Rafailidis PI.12:139-46 Lipsky BA. The role of Pseudomonas species in patients treated with ampicillin and sulbactam for gangrenous and perforated appendicitis. Adv Ther 1995. et al. Eur J Clin Microbiol Infect Dis 1994. 6th edition. Danziger LH. Expert Opin. Berne TV. South Med J 1990. Ampicillin/sulbactam versus cefazolin or cefoxitin in the treatment of skin and skin-structure infections of bacterial etiology.13:293-8 Yellin AE. Acinetobacter species. Yanagihara K. Randomized comparison of ampicillin-sulbactam to cefoxitin and doxycycline or clindamycin and gentamicin in the treatment of pelvic inflammatory disease or endometritis.161:303-7 Walker AP.17 (Suppl 3):S15-18. gentamicin plus clindamycin in the treatment of intraabdominal infections: a preliminary report. et al. Toxicol. editors. et al. Study Group of Intraabdominal Infections.5:63-7 Schutz W. Allewelt M. Hemsell PG. 81. 91. 70. et al. Dralle H. Gibbons GW.71:816-20 77. Expert Opin. Hammill HA. Comparison of ampicillin/sulbactam plus aminoglycoside vs. Wilson RF.30(Suppl 1):20A-30A Collins MD. Perri M. et al. Randomized comparative trial with ampicillin/sulbactam versus cefamandole in the therapy of community acquired pneumonia. p. Moxifloxacin vs ampicillin/sulbactam in aspiration pneumonia and primary lung abscess. Mariano N. 2005. 86. Toxicol. Dajani AS. Hammill HA.8(Suppl 5):S583-8 Holzheimer RG. Drugs 1988. Newton E. Pollak R. Berendt AR. J Infect 2008. Deery HG. et al. Hu XH. et al.127:1276-82 67. Surg Gynecol Obstet 1985. ampicillin plus clindamycin plus aminoglycoside in the treatment of intraabdominal infections in children.56:432-6 Williams D.45:995-9 Ott SR. Lorenz J.83:998-1004 Martens MG. The Multicenter Group. Boghossian J.67:1829-49 Urban C. Reyes MJ. Dajani A.6(Suppl):S55-9 Naber KG. 83. In: Mandell GL. Frantzeskaki F. 98. Efficacy and safety of high-dose ampicillin/sulbactam vs. Efficacy of a beta-lactamase inhibitor combination for serious intraabdominal infections. Faro S. et al. Hemsell DL. J Int Med Res 2001.12(Suppl 4):189S-94S Martens MG. APMIS Suppl 1989.39:885-910 Talan DA.31:464-71 Grayson ML. Diagn Microbiol Infect Dis 1989. Treatment of acute pelvic inflammatory disease in the ambulatory setting: trial of cefoxitin and doxycycline versus ampicillin-sulbactam. et al. Use of ampicillin/sulbactam and sultamicillin in pediatric infections: a re-evaluation. Intern Med 2006. et al. colistin as monotherapy for the treatment of multidrug resistant Acinetobacter baumannii ventilator-associated pneumonia. Clin Infect Dis 2002. 85. et al.6:277-91 Wilson SE. et al. Crombleholme WR. Fukuda Y. et al. Sultamicillin in the treatment of urinary tract infections. 2002 Kosseim M. Mandell.167:448-51 Cisneros JM. Faro S. Clin Infect Dis 2004. 87. 92. Does initial choice of antimicrobial therapy affect length of stay for patients with complicated intra-abdominal infections? Am Surg 2005. Multidrug-resistant Acinetobacter infections: an emerging challenge to clinicians. 95. Ampicillin/sulbactam: current status in severe bacterial infections. Croce MA. Philadelphia: Elsevier Churchill Livingstone.36:23-30 Wood GC. Ampicillin/sulbactam and cefoxitin in the treatment of cutaneous and other soft-tissue abscesses in patients with or without histories of injection drug abuse. Clinical comparative study of sulbactam/ ampicillin and imipenem/cilastatin in elderly patients with community-acquired pneumonia. Obstet Gynecol 1994. Antibiotic therapy in intra-abdominal infections–a review on randomised clinical trials. Douglas and Bennett’s principles and practice of infectious disease.35:1651-6 Allen DM. (2009) 5(9) 1111 . APMIS Suppl 1989. Diagnosis and treatment of diabetic foot infections. et al. 2632-6 Jain R. Pachon J. 97.18:683-93 Harkless L. Heard MC. Surg Infect (Larchmt) 2005.217:115-21 A randomized controlled trial of ampicillin plus sulbactam vs. J Int Med Res 2002. J Infect Dis 1993. Drug Metab. Nichols RL. Ronald A. Experience with ampicillin/ sulbactam in severe infections. 82. 79. 93. Rev Infect Dis 1986. Sulbactam/ampicillin versus cefoxitin for uncomplicated and complicated acute pelvic inflammatory disease. Drug Metab. Heseltine PN. clinical findings. 80. Clin Infect Dis 2000. 78. 94. Summanen PH. Infection 2008. Bacteremia due toAcinetobacter baumannii: epidemiology. Use of ampicillin/sulbactam versus imipenem/cilastatin in the treatment of limb-threatening foot infections in diabetic patients. et al. Comparison of ampicillin-sulbactam and imipenem-cilastatin for the treatment of acinetobacter ventilator-associated pneumonia. Habershaw GM. Ann Pharmacother 2004. Kim KS. Wittenberger R. et al. et al. 75. 72.6:27-40 88.5:57-62 Chan JC. Int J Antimicrob Agents 1996.83:408-13 Centers for Disease Control and Prevention. Pediatr Infect Dis J 1998. Effect of sulbactam on infections caused by imipenem-resistant Acinetobacter calcoaceticus biotype anitratus. Downloaded from informahealthcare. randomized study comparing efficacy and safety of intravenous piperacillin/tazobactam and ampicillin/sulbactam for infected diabetic foot ulcers. Ann Surg 1993. Antimicrob Agents Chemother 1991. 74. Sultamicillin versus trimethoprim/sulfamethoxazole in the treatment of urinary tract infections.com by HINARI on 10/08/10 For personal use only. Dolin R. Ampicillin/sulbactam versus clindamycin in the treatment of postpartum endomyometritis. 84. Drugs 2007. 73. Turpin RS. 90. Efficacy and safety of sultamicillin (750 mg bid) compared with amoxycillin/clavulanate (625 mg tid) in patients with umcomplicated urinary tract infections. 89. Ioannidou EN. Zervos MJ.34:1425-30 Betrosian AP. 76. Bennett JE. 71. Sexually transmitted diseases treatment guidelines.35(Suppl 7):39-42 McGregor JA. Go E. 68. Xanthaki A. Finegold SM. discussion S20-11 Kawada Y. 96. Seki M. Eur J Med Res 2001. et al. Chest 2005. Sulbactam/ampicillin versus metronidazole/ gentamicin in the treatment of post-cesarean section endometritis.29:257-69 Kanra G. Falagas ME. An open-label. 69. Hanes SD.Betrosian & Douzinas monotherapy for treatment of mild-to-moderate aspiration pneumonia in elderly patients. Clin Infect Dis 1994.
Hanes SD. Drug Metab. et al. Efficacy of sulbactam alone and in combination with ampicillin in nosocomial infections caused by multiresistant Acinetobacter baumannii. Comparative study of sultamicillin and amoxicillin-clavulanate: treatment of acute otitis media. et al. Lee WS. Athens. Ariza J. Wood GC. Drug excretion in human breast milk: principles. Antimicrobial drug susceptibility of clinical isolates of Acinetobacter species (A. Rybak MJ.5:1-66 110. E-mail: abetrosian@gmail. Nosokomial multi-dryg resistant Acinetobacter baumannii bloodstream infection: risk factors and outcome with ampicillin-sulbactam treatment.23:590-595 Expert Opin. 20 Papadiamantopoulou Street. Pharmacokinetics of ampicillin and sulbactam in pregnancy. Toxicol. and genospecies 6. Manrique AE.61:1369-75 107.22:1026-32 99. J Antimicrob Chemother 1998. Pharmacotherapy 2001. J Antimicrob Chemother 2008. Drug Metab. haemolyticus.21:142-8 105. resistance and outcomes of Acinetobacter baumannii becteremia treated with imipenem-cilastatin or ampicillin-sulbactam. genospecies 3.38:2055-8 108. J Int Med Res 1992. Jellison TK. Cherek DR. Koklu S. Corbella X. Bawdon R.21:58-62 102. Lin ML. Toxicol. Epidemiology. Clin Pharmacokinet 1980. Croce MA.54:32-8 106. Evgenidion Hospital. Levy CE.168:667-73 109. pharmacokinetics and projected consequences. et al.20(Suppl 1):24A-30A 111. Severe nosocomial infections with imipenem-resistant Acinetobacter baumannii treated with ampicillin/ sulbactam. Athens University. (2009) 5(9) . Spohr M. Clin Infect Dis 2002.34:1425-30 Affiliation Alex P Betrosian† MD & Emmanuel E Douzinas MD †Author for correspondence Critical Care Consultant. Wilson JT. et al.33:1617-19 104. Chan KH. Fax: +30 210 7208100. Clin Infect Dis 1996. Borer A. Arguello A. 3rd Department of Critical Care.Ampicillin-sulbactam and prognostic features. Riesemberg K. An open comparative study of the efficacy and safety of sultamicillin versus cefaclor in the treatment of acute otitis media in children. Ann Pharmacother 2004.com 1112 Expert Opin. Int J Antimicrob Agents 2003. A. Pediatr Infect Dis J 1993. et al. Ampicillin/sulbactam compared with polymyxins for the treatment of infections caused by carbapenem-resistant Acinetobacter spp. Probable sulbactam/ampicillin-associated prolonged cholestasis.20(Suppl 1):31A-43A 112. et al.baumannii. Costa SF. Brown RD. Ardanuy C. et al. Prado GV. Chamberlain A. Levin AS. Smolyakov R. In vitro activities of beta-lactam antibiotics alone and in combination with sulbactam against Gram-negative bacteria. Biolcati AH. J Hosp Infect 2003. Bluestone CD. An open non-comparative pilot study of the safety and efficacy of oral sultamicillin in the treatment of mild to moderate upper respiratory tract infections in children.12:24-8 100. Tan LS. Wang FD. Mckinnon PS. Traub WH. et al. Asil M.com by HINARI on 10/08/10 For personal use only. 101. et al. 11528. Oliveira MS. Int J Antimicrob Agents 2004. Greece Tel: +30 210 7208116.42:793-802 103. Koksal AS. Am J Obstet Gynecol 1993. Downloaded from informahealthcare. J Int Med Res 1992. Antimicrob Agents Chemother 1997. Comparison of ampicillin-sulbactam and imipenem-cilastatin for the treatment of acinetobacter ventilator-associated pneumonia. White S.